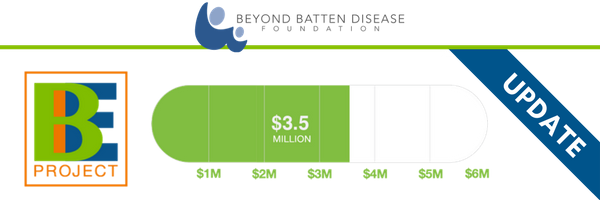
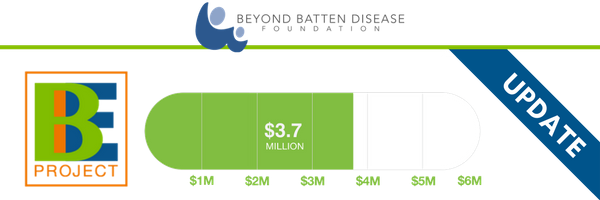
Brineura Approval Invigorates Research, Awareness About Other Batten Treatments

Soon after the first-ever therapy for Batten disease was approved by the U.S. Food and Drug Administration (FDA) in April 2017, Danielle Kerkovich, principal scientist at the Beyond Batten Disease Foundation, started fielding questions from families wondering if their child could take the new treatment.
Kerkovich had to tell parents that they, unfortunately, could not. That’s because the Beyond Batten Disease Foundation works with kids who have the CLN3 form of Batten disease. Brineura (cerliponase alpha), developed by the pharmaceutical company BioMarin, can’t be used for CLN3 — it was designed for patients with the CLN2 form of the disease.





 Listen to BBDF co-founder, Charlotte Benson as she discusses coping with a child’s terminal illness on The TKO Show with Kara Ro.
Listen to BBDF co-founder, Charlotte Benson as she discusses coping with a child’s terminal illness on The TKO Show with Kara Ro.