Batten-1 (Formerly BBDF 101)
A treatment to slow the progression of juvenile Batten (CLN3) disease
2023
- New Advance to the Theranexus and BBDF Batten-1 Program for Juvenile Batten Disease (CLN3)
- Theranexus, BBDF and Cardiff University Present Their Novel Research on Batten-1 and Batten Disease at WorldSymposium 2023
2022
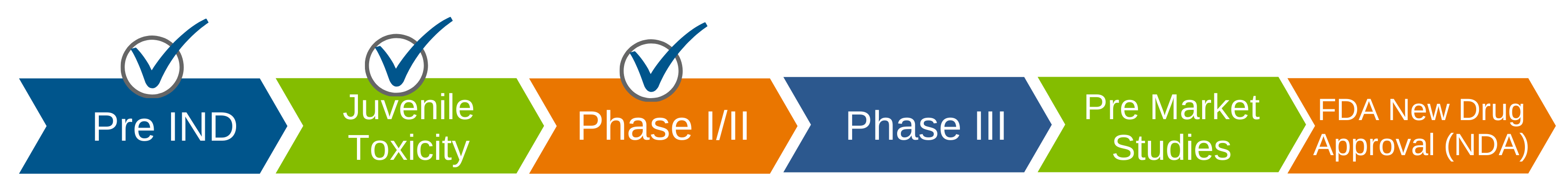
- BBDF 101 Phase l/ll initiated
- Theranexus announced that it is now, more than ever, committed to support patients with rare neurological disorders by focusing all its research efforts on these diseases, starting with CLN3. In this context, the project with Beyond Batten Disease Foundation (BBDF) becomes their flagship project and they have decided to dedicate all resources and efforts to make this project a success to benefit of the CLN3 disease community. Following the initiation of the ongoing Phase l/ll clinical study involving 6 young adult patients, Theranexus and BBDF are in the process of finalizing discussions with the FDA to initiate Phase III.
- In April, BBDF and Theranexus made the decision to switch from BBDF 101, a combination of miglustat and trehalose, to Batten-1, a new patient friendly formulation of miglustat alone. This decision was based on recent results with miglustat in CLN3 disease showing excellent efficacy of this drug in the models.
2021
- BBDF 101 Wins FDA Investigational New Drug Approval (IND).
2020
- The FDA has awarded Orphan Drug and Rare Pediatric Disease designations to Beyond Batten Disease Foundation for BBDF-101
Press Release »
2019
- Theranexus, a publicly-traded European pharmaceutical company, has committed the $20 million and expertise necessary to complete the clinical trial and commercialization for our drug discovery, BBDF-101.
Press Release »
2018
Preparing for clinical trial success
- Camargo Regulatory Strategists join BBDF to bring BBDF-101 to the U.S. Federal Drug Administration (FDA) for permission to enter into a clinical trial.
- After almost 9 years and over $23 million invested in overall research, the teams from BBDF and Camargo, along with key opinion leaders in Batten disease from University of Rochester, University Medical Center Hamburg-Eppendorf, Texas Children’s Hospital and BDSRA, meet with the FDA for BBDF 101’s Pre Investigational New Drug (PreIND) meeting. This is the first formal step in clinical trial development.
2016
- NRI researchers learn that, independent of Drug #1, Drug #2 clears a subset of waste material and inhibits the harmful effects of chronic inflammation in the brain, resulting in additional unexpected benefits of the drug combination.
- Evotec scientists conduct hundreds of experiments to determine the correct dosage for each drug independently, and in-combination with one another.
2014
100s of experiments for dosing, safety and efficacy
- $2.2 million in BBDF-funding to German pharmaceutical-grade contract research organization, Evotec, leads to the prioritization of Drug #1, the most promising compound with little to no side-effects.
- Evotec and others conduct 125 experiments to determine that the best way for Drug #1 to reach the brain is through intravenous administration.
- Leveraging BBDF’s investments with over $3 million in nonprofit and NIH-funding leads to the addition of Drug #2. Drug #2 enhances the ability of Drug #1 to reach the brain, therefore, creating the combination therapy BBDF 101.
2012
- NRI investigators, together with international collaborators, demonstrate TFEB activated lysosome production is safe and effective at clearing accumulated waste material across multiple healthy and diseased animals throughout their development.
- Three years and an additional $1.8 million in BBDF-funding for labor-intensive searches and 100s of experiments lead to the identification of 6 potential drugs to treat CLN3 disease via TFEB activation.
2008
Finding a solution to the problem
and identifying potential drugs for treatment
- Beyond Batten Disease Foundation (BBDF) opens its doors and begins looking for a solution to fix what goes wrong in Batten-affected cells. Children with Batten have cellular waste centers (lysosomes) that work, but they work inefficiently. Over time, brain cells fill with toxic waste material and die.
- Transcription Factor –EB (TFEB), a master controller of lysosome production is discovered. If researchers can activate TFEB in Batten-affected cells, maybe each cell could clear itself, staving off its own death and slowing disease.
- BBDF grants TFEB discovery team $2.5 million to join the Jan and Dan Duncan Neurological Research Institute (NRI) at Texas Children’s Hospital to apply TFEB activation to juvenile Batten (CLN3 disease). Housed on the largest medical center campus in the world, researchers have access to unprecedented resources.

2 thoughts on “Batten-1”