“Ten years ago, I would have thought that a world without genetic killers like Batten, cystic fibrosis and hundreds of others was a dream. Beyond Batten Disease Foundation is making this dream a reality.”
-Mehmet Oz, MD, The Dr. Oz Show
What is Genetic Testing or Screening?
Genetic Testing is the direct examination of one’s DNA to identify specific gene mutations (mistakes) that are known to lead to diseases like Batten, Cystic Fibrosis, Muscular Dystrophy and others.
Why is Genetic Testing important?
Genetic testing plays a critical role in accurately diagnosing diseases early enough to prevent an all too common time-consuming, expensive and emotionally exhausting diagnostic odyssey, to prevent treatment errors resulting from misdiagnoses, and to ensure patients and their families receive the support and educational services they need.7,13 Genetic testing can also be useful in family planning following the birth of an affected child.
Beyond Batten Disease Foundation’s contribution to Rare Disease Genetic Testing
In the fall of 2008, Beyond Batten Disease Foundation founder Craig Benson and founding director, Mark Chandler, PhD, approached National Center for Genome Resources researchers Stephen Kingsmore and Callum Bell with a challenge: to create a precise, inexpensive, genetic test capable of detecting hundreds of devastating genetic diseases, one that could be made widely available to prevent the emotional and financial consequences associated with lengthy diagnostic odysseys.
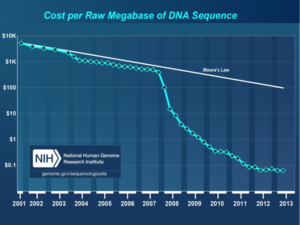
Courtesy: National Human Genome Research Institute.
They believed that recent improvements in DNA sequencing and robotic and sensor technologies, together with the dropping costs of these technologies made it the perfect time to develop such a test (Cost per Raw Megabase of DNA Sequence).
What does it mean to be a “carrier?”
Normally, every person has two copies of every gene, one inherited from each parent. Many (but not all) genetic diseases require two defective genes in order to develop; if one inherits one normal and one mutated gene, he or she does not have the disease but is a carrier and can pass the mutated gene onto their children (Autosomal Recessive).
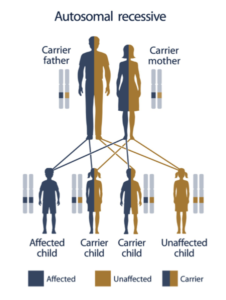
Courtesy: U.S. National Library of Medicine
Why screen for rare diseases? They’re rare and don’t affect many people.
While the incidence of each individual rare disease is low, these numbers add up: an estimated 25-30 million people in the United States are afflicted with one of more than 6,800 rare diseases, 80% of which are genetic. The worldwide incidence is estimated to be 350 million people – more than AIDS and cancer combined.11
Why screen for rare diseases for which no treatment is available?
In order to bring a drug to market for any disease, the U.S. FDA requires proof of concept studies in reliable animal models of the disease, valid comparisons between animal models and affected patients, and well-defined methods for assessing individual treatment responses. Without properly diagnosed patient pools to study, none of these criteria can be met inhibiting drug development.
The History of Genetic Screening
Newborn screening began in the 1960s when Dr, Robert Guthrie developed a blood test for newborns that could detect phenylketonuria (PKU), a condition which leaves infants unable to break down the amino acid phenylalanine, causing serious symptoms like seizures and mental retardation.8 Fortunately, PKU is treatable with a restricted diet.
The first large-scale carrier screening came in the early 1970s, a time when 1 in 158 infants born on the island of Cyprus were at risk for beta thalassemia, a life-threatening blood disorder. To address this issue, the government and Orthodox Church established a policy of mandatory carrier screening and counseling prior to starting a family.2 As a result, the disease has virtually disappeared from the Island.1
Rabbi Joseph Eckstein pioneered a similar program offering genetic screening to members of the Jewish community to eliminate Tay-Sachs and other genetic disorders common in the Jewish community. One in 27 Ashkenazi Jews are carriers for Tay-Sachs, but incidence in North America has dropped 90% since the start of the screening program.5,6,9
The Current State of Genetic Testing
Since the start of newborn screening for PKU, scientists have developed more tests to screen newborns for up to 60 different disorders. 6 However, states vary in the number (30-60), types of conditions, and quality of their testing protocols.
Carrier screening is available for more than 60 of the estimated 5,200 rare genetic diseases, but individual tests can range from $200 to $1,200; making comprehensive preconception carrier screening cost-prohibitive for even the wealthiest families.6,12 Importantly, most affected infants are from families with no history of the disorder, so making carrier screenings more widely accessible is key to reducing the incidences of these genetic disorders.
Where is Beyond Batten Disease Foundation’s Screen Now?
 Kingsmore and his colleagues at the National Center for Genome Resources in Santa Fe, New Mexico rose to BBDF’s challenge, and in January 2011 they published the article “Carrier testing for severe childhood recessive diseases by next-generation sequencing” in Science Translational Medicine, one of the highest-ranking among more than 28,000 scientific and technology journals. Researchers demonstrated the test’s ability to detect mutations in 7,717 regions from 437 target genes that are responsible for 448 diseases. Amazingly, the screening is able to detect these mutations with an accuracy of 100%, the highest reported of any DNA testing platform to date.3,4 In 2012, Time magazine named the technology one of the Top Ten Medical Breakthroughs of the year.12 The development team has since moved to Children’s Mercy Hospital (CMH) in Kansas City where the test, now named “TaGSCAN” (for Targeted Gene Sequencing and Custom Analysis) has been clinically validated to identify 750 devastating rare diseases, including the juvenile (CLN3) form and other forms of Batten disease. To see a list of targeted genes go to http://www.childrensmercy.org/Unlinked_Content/TaGSCAN_Diagnostic_Test/. The test is now available and can be ordered by any qualified physician who suspects his or her patient has a genetic illness.
Kingsmore and his colleagues at the National Center for Genome Resources in Santa Fe, New Mexico rose to BBDF’s challenge, and in January 2011 they published the article “Carrier testing for severe childhood recessive diseases by next-generation sequencing” in Science Translational Medicine, one of the highest-ranking among more than 28,000 scientific and technology journals. Researchers demonstrated the test’s ability to detect mutations in 7,717 regions from 437 target genes that are responsible for 448 diseases. Amazingly, the screening is able to detect these mutations with an accuracy of 100%, the highest reported of any DNA testing platform to date.3,4 In 2012, Time magazine named the technology one of the Top Ten Medical Breakthroughs of the year.12 The development team has since moved to Children’s Mercy Hospital (CMH) in Kansas City where the test, now named “TaGSCAN” (for Targeted Gene Sequencing and Custom Analysis) has been clinically validated to identify 750 devastating rare diseases, including the juvenile (CLN3) form and other forms of Batten disease. To see a list of targeted genes go to http://www.childrensmercy.org/Unlinked_Content/TaGSCAN_Diagnostic_Test/. The test is now available and can be ordered by any qualified physician who suspects his or her patient has a genetic illness.
Until now, the average patient with a rare disease would see 8 physicians and receive 2 to 3 misdiagnoses over the course of 7.6 years before being correctly diagnosed. Since its launch, the test has helped thousands of children and families touched by rare disease. More recently, the test has been licensed to PerkinElmer, one of the world’s largest life sciences and diagnostic services companies. In the future, BBDF will be entitled to receive royalties from commercial sales of the test.
To learn more about or to order genetic testing for a patient suspected of having a genetic illness, go to Lysosomal Storage Disease Treatment centers listed on our Diagnosis page. Additional testing is available through Invitae including carrier testing.
References
- Angastiniotis M, Kyriakidou S, Hadjiminas M. How thalassaemia was controlled in Cyprus. World Health Forum. 1986. Vol 7:291-297.
- Angastiniotis MA, Hadjiminas MG. Prevention of thalassaemia in Cyprus. Lancet. 1981. Feb 14:1(8216):369-71.
- Bell CJ, Dinwiddie DL, Miller NA, et al. Carrier testing for severe childhood recessive diseases by next-generation sequencing. Sci Transl Med. 2011 Jan 12;3(65):65.
- Couzin-Frankel J. New high-tech screen takes carrier testing to the next level. Science 2011 Apr 1;332(6025):36.
- Eckstein J and Katzenstein H. The Dor Yeshorim story: community-based carrier screening for Tay-Sachs disease. Adv Genet 2001. 44:297-310.
- Genetics and Family History. About Newborn Screening. Baby’s First Test [Internet]. Rockville (MD): Maternal and Child Health Bureau, Health Resources and Services Administration (HRSA), grant no. U36MC26509. [Accessed February 17, 2017]. Available from: http://www.babysfirsttest.org.
- Genomic Resource Centre [Internet]. World Health Organization United Nations. [Accessed February 7, 2017]. Available from: http://www.who.int/genomics/elsi/gentesting/en/index.html.
- Guthrie, R.: Blood screening for phenylketonuria, J.A.M.A., 1961. Nov 178:863.
- Kaback M, Lim-Steele J, Dabholkar D, et al. Tay-Sachs disease-carrier screening, prenatal diagnosis, and the molecular era. An international perspective, 1970 to 1993. The International TSD Data Collection Network. J.A.M.A. 1993. Nov 17;270(19):2307-15.
- Rare Diseases: Common Issues in Drug Development [Internet]. U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research, and Center for Biologics Evaluation and Research. [Accessed February 7, 2017]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM458485.pdf
- RARE facts and Statistics [Internet]. The Global Genes Project. Team RARE and RARE Project. [accessed February 7, 2017]. Available from: https://globalgenes.org/rare-diseases-facts-statistics/.
- Rochman B. “The DNA Dilemma: A test that could change your life” December 24, 2012. http://www.time.com/time/magazine/article/0,9171,213532,00.html.
- Shire HGT. Rare Disease Impact Report [Accessed February 7, 2017]. 2013 Apr. Available from: https://globalgenes.org/rare-disease-impact-report/
